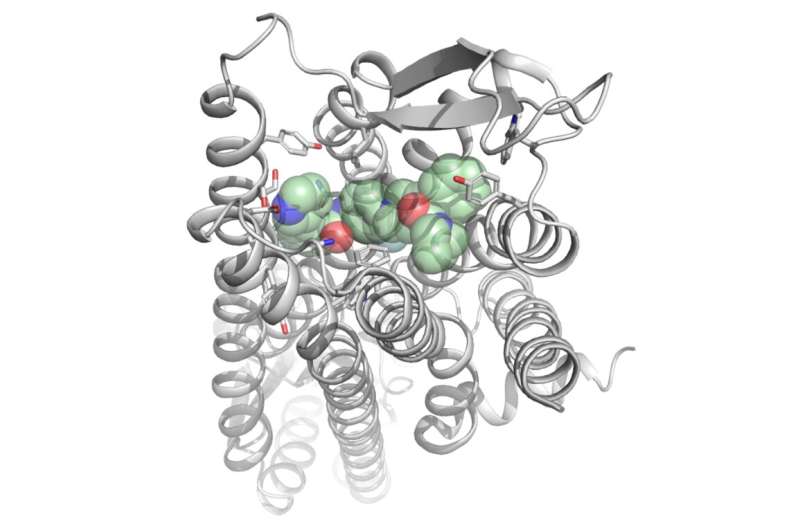
In order for a drug to be effective at the right places in the body, it helps if scientists can predict as accurately as possible how the molecules of that drug will interact with human cells. In a joint research project, scientists from Collaborative Research Centre 1423 at Leipzig University and the Chinese Academy of Sciences in Shanghai have succeeded in elucidating such a structure, namely that of the neuropeptide Y receptor Y2 with one of its ligands. This is the first time that a molecular blueprint for this receptor is available, which will enable the development of tailor-made new drugs, for example to treat epilepsy or cardiovascular diseases. The researchers’ findings have now been published in Nature Communications.
The Y2 receptor plays an important role, especially in the peripheral nervous system and in the brain, as it is considered one of the ‘satiety receptors.’ It also plays a role in epilepsy as well as in cardiovascular diseases. If these diseases are to be treated with drugs that block the Y2 receptor, it is important to ensure that the drug can target this receptor precisely and exclusively, because some closely related receptors would have exactly the opposite effect. When developing novel drugs, it is therefore essential to obtain highly targeted compounds and to have precise knowledge of their molecular properties.
Researchers led by Professor Annette Beck-Sickinger and Dr. Anette Kaiser at Leipzig University have succeeded in showing on the molecular level how substances can block the Y2 receptor. Working with their colleagues in Shanghai, they were able to explain the crystal structure with a bound ligand, validate it by means of numerous biochemical investigations, and transfer it to other systems. The new study also reveals that Y2 receptor blockers bind differently than comparable molecules at the closely related Y1 subtype. This will facilitate further knowledge-based development of selective compounds at both receptors.
Source: Read Full Article
