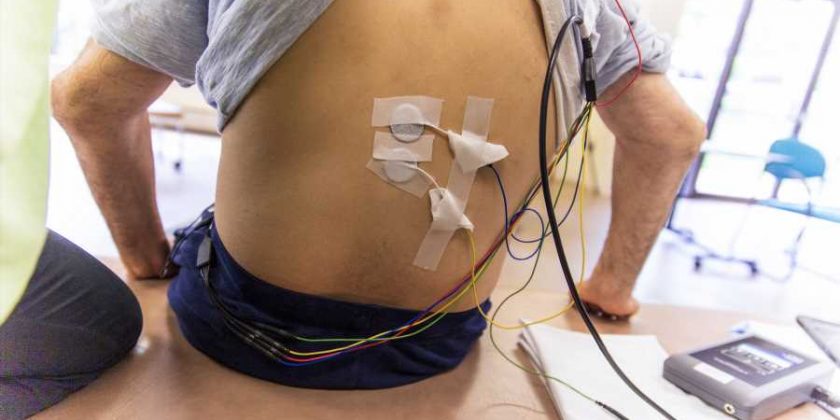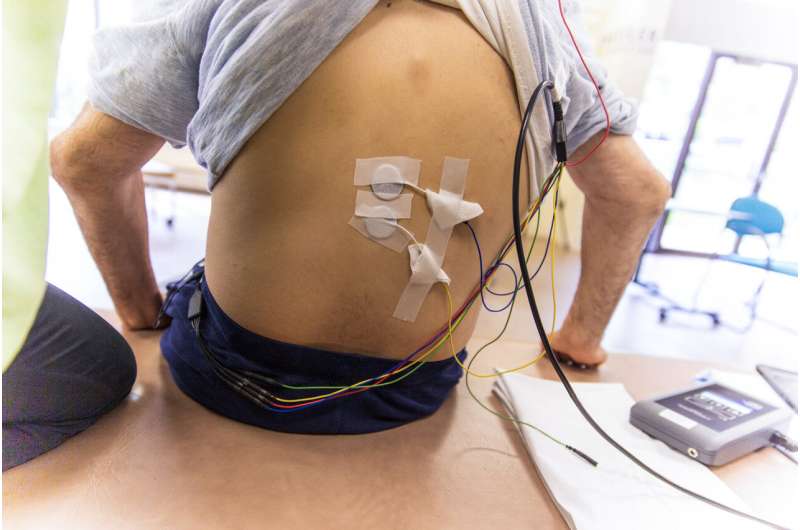
Researchers from Kessler Foundation and Kessler Institute for Rehabilitation (collectively termed “Kessler”) have conducted the first prospective study to assess whether transcutaneous spinal stimulation (TSS) interacts with implanted intrathecal baclofen (ITB) pump delivery systems for managing spasticity. Their article, “Transcutaneous spinal stimulation in patients with intrathecal baclofen pump delivery system: A preliminary safety study,” was published December 21, 2022, in Frontiers in Neuroscience.
The pilot study was conducted at the Tim and Caroline Reynolds Center for Spinal Stimulation by John Lopez, DO, Gail Forrest, Ph.D., Einat Engel-Haber, MD, Brittany Snider, MD, Kam Momeni, Ph.D., Manikandan Ravi, MS, and Steven Kirshblum, MD.
Due to a lack of adequate data on how spinal stimulation affects intrathecal medication delivery, individuals with pump delivery systems are often excluded from TSS trials. In this study, Kessler team members tested their hypothesis that TSS would not interfere with the ITB pump system. The five study participants were adults with chronic traumatic spinal cord injury, each with an anteriorly implanted ITB pump delivery system (Medtronic SynchroMed II).
Each participant underwent two trials of TSS: the first to establish intensity of lower thoracic stimulation as measured by surface electromyography of the lower extremity muscles, and the second to deliver 30 minutes of moderate-intensity stimulation. To monitor pump function, the pump was interrogated before, during, and after stimulation.
Results revealed no evidence for pump dysfunction as measured by pump log evaluations. Interference of the communication between the interrogator and the pump was evidenced by lapses in log transmission during TSS, most likely due to transient electromagnetic interference. One participant had an adverse event that was likely unrelated to the TSS, but rather related to a bladder issue and underlying urinary tract infection.
“Based on our preliminary findings, individuals with implanted baclofen pumps may be considered for TSS studies involving the low thoracic spine,” said Dr. Lopez, staff physiatrist at Kessler Institute for Rehabilitation. “While communication between the pump and its interrogator may be briefly affected, there was no evidence that medication delivery was disrupted.”
Given the potential benefits of TSS in the population with spinal cord injury, further research is warranted, according to Dr. Kirshblum, chief medical officer of Kessler Institute for Rehabilitation and Kessler Foundation and director of Spinal Cord Injury Services at Kessler Institute for Rehabilitation. “We need to study the effects of various patterns and intensities of transcutaneous stimulation in patients with baclofen pumps,” he noted, “as well as the effects of repeated sessions and stimulation at multiple sites.”
More information:
John Lopez et al, Transcutaneous spinal stimulation in patients with intrathecal baclofen pump delivery system: A preliminary safety study, Frontiers in Neuroscience (2022). DOI: 10.3389/fnins.2022.1075293
Journal information:
Frontiers in Neuroscience
Source: Read Full Article