Insights that pave the way for novel therapeutic approaches to tackle cancer, fibrosis, and many age-related conditions have been uncovered by a team of scientists, including researchers from UCL.
The research, published in Nature Cell Biology and led by the Laboratory of Medical Sciences (MRC-LMS), identifies a new target for a class of drugs that selectively eliminate cells that are known to provoke inflammation.
The drugs, known as senolytics, eliminate senescent ‘zombie’ cells that have stopped multiplying in response to either cell damage or aging. Although senescent cells are inactive, they continue to release proteins that can induce inflammation in the body. Usually, the body eliminates senescent cells through the immune system. However, aging and disease can disrupt this process. Consequently, these cells can accumulate in the body, causing chronic inflammation and tissue disruption.
This further fuels the aging process and disease development, generating more senescent cells and forming a vicious cycle.
By removing senescent cells, senolytics reestablish tissue stability, improving the outcome of many age-related diseases.
Though pre-clinical studies have shown some promising results, there are currently no senolytics on the market and those in clinical trials have limitations, creating calls for more effective options to be developed.
Lead author Professor Jesus Gil (Head of the Senescence Research Group at LMS) said, “In a previous study, we showed the potential of repurposing existing drugs, but there are only limited drugs to choose from.”
“In this study, we greatly expanded our selection pool by seeking targets in over 7,000 ‘druggable’ genes. We were thrilled to reveal previously unknown vulnerabilities of senescent cells. This opens up new possibilities for treating age-related diseases.”
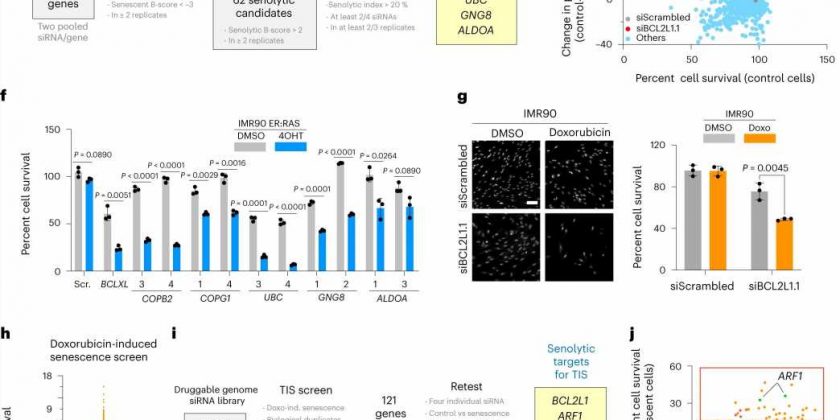
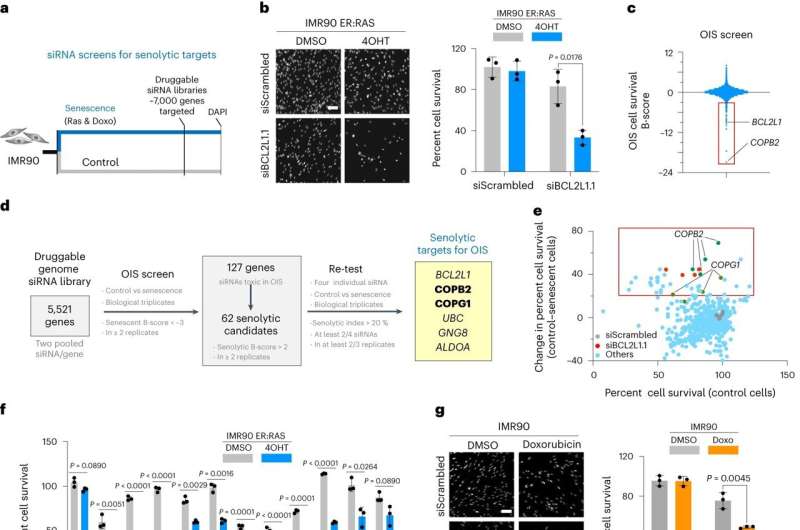
The team used an approach called RNA interference (RNAi) to assess the molecular pathways influencing the survival of senescent cells. RNAi prevents protein production by reducing gene expression.
They screened RNAi molecules targeting over 7,000 genes and selected the RNAi molecules that selectively killed senescent cells but not normal cells.
As a result, they identified a senolytic target pathway called coat protein complex I (COPI). Inhibiting this pathway, which is responsible for carrying proteins in the cells, led to the death of senescent cells.
They also showed that targeting this pathway improved outcomes in mouse models of cancer and fibrosis.
Co-author Professor J.P. Martinez-Barbera (UCL Great Ormond Street Institute of Child Health), said, “When we tested these senolytics in preclinical models of a pediatric brain tumor called craniopharyngioma, we were amazed to see that the majority of the senescent cells had been killed.”
“These results have encouraged us to explore these drugs further as potential treatments against these aggressive tumors.”
Building on these findings, the researchers explored drug candidates that target the COPI pathway. Although some existing drugs directly interfere with the COPI pathway, they have limited effectiveness because they only have a short lifespan in the bloodstream, so they are not considered suitable for clinical use.
The LMS team collaborated with Prof Edward Tate at Imperial College London and Myricx Bio, an Imperial spin-off, to investigate whether a different class of drugs that indirectly inhibit the COPI pathway, N-myristoyltransferase inhibitors (NMTi), could also target senescent cells. The results were positive- NMTi demonstrated potent senolytic effects, effectively improving the outcome of cancer and fibrosis in mouse models.
Lead author Prof Jesus Gil said, “This work defines a novel class of senolytic drugs that expand the possibilities to treat a wide range of diseases associated with senescence including cancer, idiopathic pulmonary fibrosis (IPF) or non-alcoholic steatohepatitis (NASH).”
The study opens up a new avenue of opportunity to develop drugs that can attack significant diseases such as cancer, fibrosis, and other aging-related conditions. The team is now looking ahead to further research and potential clinical trials, representing a promising advancement in the quest for healthier aging.
More information:
Domhnall McHugh et al, COPI vesicle formation and N-myristoylation are targetable vulnerabilities of senescent cells, Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01287-6
Journal information:
Nature Cell Biology
Source: Read Full Article