Drug addiction is characterized by compulsive drug use among individuals despite adverse consequences underlying such tendencies. However, the specific neural circuits underlying the mechanisms of addictive behavior remain unidentified. In a new study now published in Science Advances, Yang Chen and a research team in medical sciences, biomimetic drugs, and neuroscience in China conducted several experiments with animal models to understand specific neural mechanisms behind cocaine addiction.
During the experiments, they implemented a footshock-triggered method of cocaine self-administration among the rats to assess the variability of rodent behavior during the process. The outcomes showed how rats with compulsively habitual cocaine use presented increased neural activity of the anterior insular cortex (aIC) when compared with non-compulsive rats.
Chen and colleagues recognized the chemogenic manipulating activity of neurons in the anterior insular cortex (aIC), which received inputs from the orbitofrontal cortex (OFC) during compulsive cocaine use. When they suppressed this OFC-aIC neural circuit, the rat behavior changed from triggered compulsive activity to display sensitivity. This work showed how the aIC glutamatergic neurons and the orbitofrontal cortex-anterior insular cortex facilitated a shift towards compulsive cocaine use, which can serve as a therapeutic target to prevent drug addiction.
The insular cortex of the mammalian brain
Compulsive drug addiction is characterized by persistent drug use regardless of negative consequences surrounding a user’s addictive behavior. Epidemiological studies have revealed individual differences among users during the development of drug addiction. Neuroscientists have recognized the insular cortex to be involved in a spectrum of drug addictive behavior. Interestingly, smokers with insular cortex damage can undergo smoking disruption, allowing them to discontinue smoking. However, implications of the insular cortex during compulsive drug seeking behavior remain unknown.
The insular cortex is a complex anatomical hubris in the mammalian brain, which includes an anterior insular cortex (aIC) and a posterior insular cortex (pIC). The researchers showed how the anterior insular cortex is associated with compulsive cocaine use. They demonstrated this by implementing a footshock-triggered cocaine self-administration procedure and combined the process with cluster analysis, immunostaining, fiber photometry, electrophysiology, and chemo-genetics.
The outcomes showed how the glutamatergic neurons of the anterior insular cortex and orbitofrontal cortex-anterior insular cortex regions played a key role to regulate compulsive cocaine use. The outcomes provide hitherto unidentified anatomical and functional circuits to therapeutically mediate drug addiction.
The rodent experiments
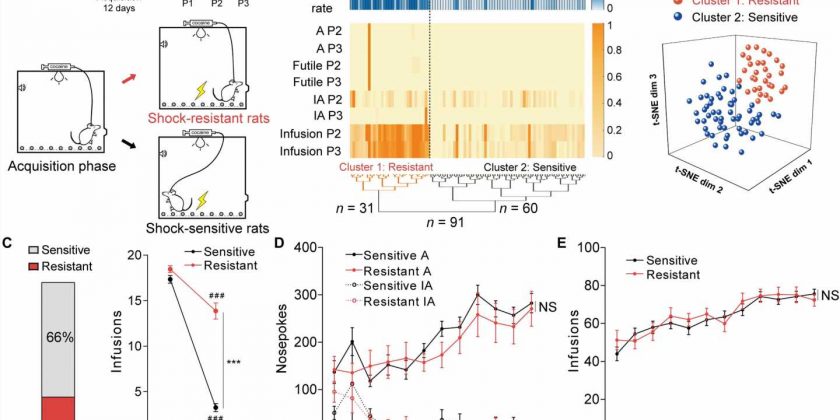
The research team conducted experiments with the rats who were trained to self-administer cocaine across 12 days. Under the conditions of freely available cocaine use, rats learned to compulsively self-administer the drug. When the team introduced three days of an hour of footshock-triggered drug administration, they observed that approximately 34% of the rats were shock resistant, while 66% were shock sensitive.
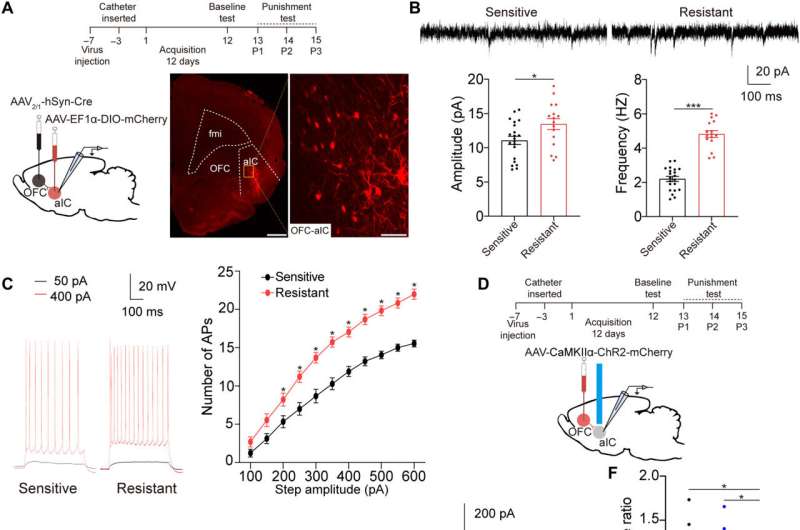
The cocaine-sensitive rats quickly reduced self-administration of the drug, while those resistant to the dose kept administering the drug despite the trigger. Further analysis showed the anterior insular cortex neurons of the shock-resistant rats to be hyperactive. The researchers performed specific neural activities to examine the activation of anterior insular neurons in the two groups of rats via trigger assays.
Investigating different proteins of interest involved in cocaine addiction
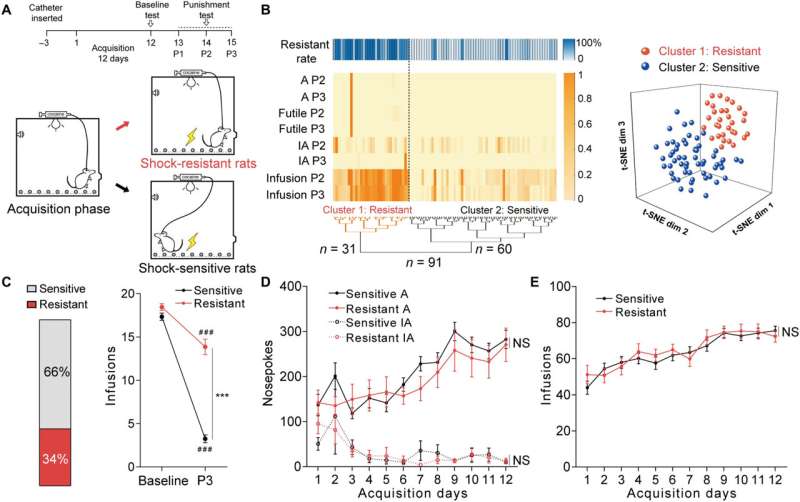
The team first studied the c-Fos protein expression specific to brain development in several nonspecific brain regions involved with drug addiction. The rats presenting with drug resistance had an increased number of c-Fos positive neurons in the anterior insular cortex, the orbital frontal cortex, nucleus accumbens and in the central amygdala, in comparison to sensitive rats.
While the team positively stained neurons for this protein of interest, they could not be differentiated between drug resistant and sensitive rats in the parietal insular cortex, indicating their exclusion during compulsive cocaine use within the specific brain regions. The team additionally confirmed the relationship between the activated anterior insular cortex neurons and compulsive cocaine use with further experiments to highlight a connection between cocaine use and hyperactivity of the neurons.
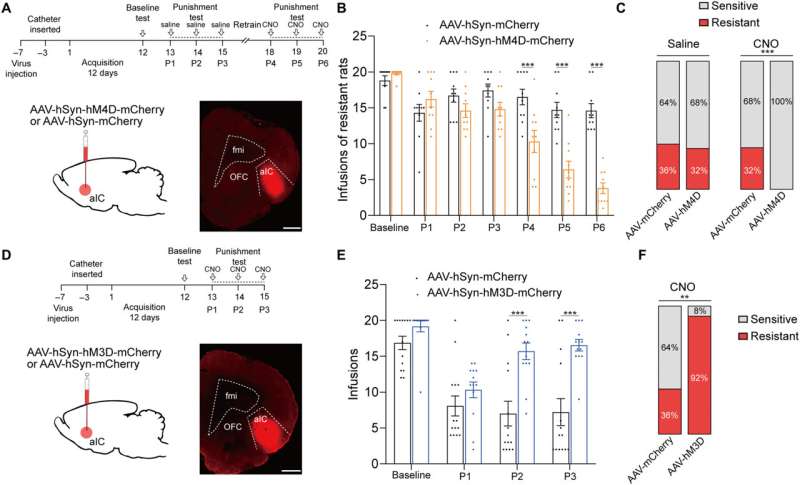
Additional experiments showed the activation of the anterior insular cortex neurons for compulsive cocaine use. During chemically induced inhibition of the anterior insular cortex, the team noted a significant reduction of compulsive cocaine use among resistant rats alongside a notable restoration of their sensitivity to triggers. However, similar inhibition of the same brain region in already sensitive rats did not affect their use of cocaine during triggers. The outcomes yet again highlighted the necessity of activating the anterior insular cortex for compulsive cocaine use.
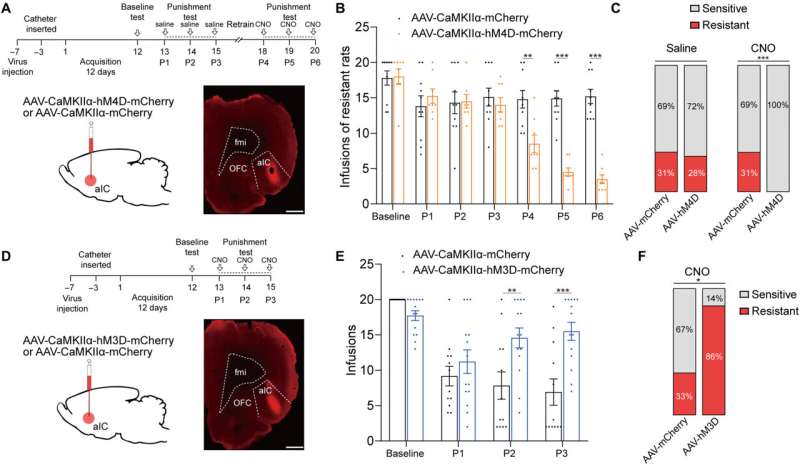
The role of the anterior insular cortex (aIC) glutamatergic neurons and the OFC-aIC axis
Since previous investigations linked the aIC (anterior insular cortex) neurons with glutamatergic properties; glutamate is the major excitatory neurotransmitter in the nervous system, the researchers speculated that this region modulates compulsive cocaine use. They specifically tested this by inhibiting the characteristic neuron subset with a chemogenic assay, which reduced compulsive cocaine use, while inhibiting the aIC glutamatergic neurons among rats already sensitive to cocaine did not alter their drug use. The neuron subtype bidirectionally regulated compulsive cocaine use among the animal models.
Further studies showed how the anterior insular cortex received direct inputs from the orbital frontal cortex (OFC-aIC axis). The team used a viral vector system to study the implications of the OFC-aIC pathway during compulsive cocaine use between sensitive and resistant rats. The outcomes highlighted the significance of activating the neural circuit for compulsive drug use.
Outlook
In this way, Yang Chen and colleagues used animal models to show the gating role of glutamatergic neurons of the anterior insular cortex (aIC) and the orbital frontal cortex- anterior insular cortex (OFC-aIC) circuit, during the regulation of compulsive cocaine use. The outcomes highlighted how patients with insula damage could easily quit smoking. The findings can add to the understanding of compulsive drug addiction and the design of related therapies to attenuate the tendency.
More information:
Yang Chen et al, An orbitofrontal cortex–anterior insular cortex circuit gates compulsive cocaine use, Science Advances (2022). DOI: 10.1126/sciadv.abq5745
Billy T. Chen et al, Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking, Nature (2013). DOI: 10.1038/nature12024
Journal information:
Science Advances
,
Nature
Source: Read Full Article