Radionuclide therapy has proven successful in delaying the growth of disseminated tumor cells (DTCs) in early-stage breast cancer in a small animal model, suggesting its use as a potential adjuvant therapy for retarding the proliferation of DTCs. As reported in the January issue of the Journal of Nuclear Medicine, the alpha-particle-emitting radiopharmaceutical 223RaCl2 not only impacts cells directly hit by radiation but also has significant effects on cells outside of the radiation field (i.e., bystander cells).
Breast cancer is the most common cancer diagnosed in women in the United States. While survival rates for women are high, approximately 20 percent of five-year survivors ultimately develop metastatic disease five to ten years after treatment. The formation of metastases involves circulating tumor cells that shed from the primary tumor and gain access to the circulatory system. These DTCs may sustain active proliferation and develop into macrometastases or may remain dormant for years before becoming active.
“With a renewed interest in therapy with alpha-particle emitters and their potential for sterilizing DTCs, our study sought to determine whether bystander effects play a role in 223RaCl2 therapy and, if so, whether they can be leveraged to treat DTCs before disease progression,” noted Roger W. Howell, Ph.D., and co-authors at Rutgers New Jersey Medical School, University of Florida and University of Gothenburg.
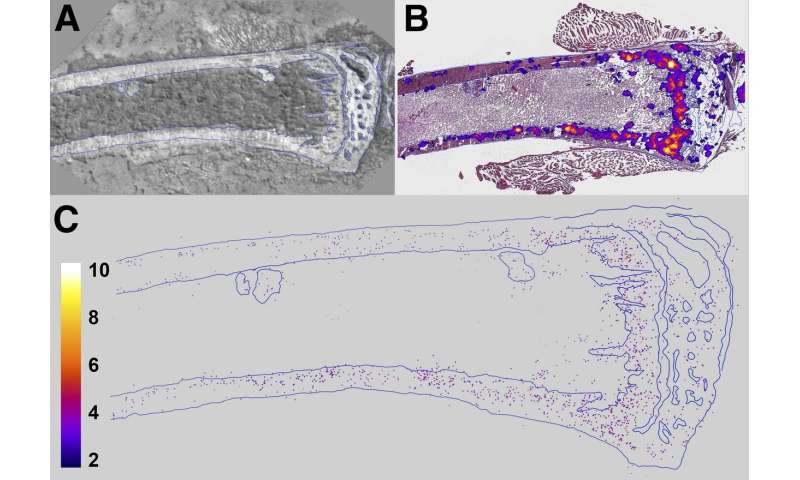
In the study, female mice were administered 0, 50 or 600 kBq/kg of 223RaCl2 to create bystander conditions prior to tumor cell inoculation. After 24 hours, mice were inoculated with either estrogen receptor-positive human breast cancer cells or triple-negative (estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2-negative) human breast cancer cells into the tibial marrow compartment. Bioluminescence intensity of the inoculated tumor cell populations was measured on day one and weekly thereafter.
Tumor burden analysis revealed that DTCs were present both within and beyond the range of the alpha particles emitted from 223RaCl2 in both types of breast cancer cells. Growth delays were then tracked for each group of breast cancer cells. Estrogen receptor-positive breast cancer cells responded to the 50 and 600 kBq/kg treatments with seven-day and 65-day growth delays, respectively. In contrast, the triple negative breast cancer cells demonstrated a 10-day growth delay in tumor progression for the 600 kBq/kg group. No significant difference was noted for the triple negative breast cancer cell group administered 50 kBq/kg when compared to the control group.
“The increased magnitude of the bystander effect in this study suggests that higher injected activities may better sterilize undetected dormant or slow-growing DTCs in the bone marrow micro-environment.,” said Howell and co-authors. “Thus, 223RaCl2 may potentially be an adjuvant treatment option for select patients at early stages of breast cancer.
They continued, “This study adds to the mounting evidence that radiation-induced bystander effects can play a role in in the design of future treatment plans for radiopharmaceuticals alone or combined with external-beam therapy. Furthermore, the capacity to target specific cells or tissues in a systemic manner may offer advantages over the use of external beams of radiation for eliciting therapeutic bystander responses.”
The authors of “Dose-Dependent Growth Delay of Breast Cancer Xenografts in the Bone Marrow of Mice Treated with 223Ra: The Role of Bystander Effects and Their Potential for Therapy” include Calvin N. Leung, Edouard I. Azzam and Roger W. Howell, Department of Radiology, New Jersey Medical School, Rutgers University, Newark, New Jersey; Brian S. Canter, Department of Radiology, New Jersey Medical School, Rutgers University, Newark, New Jersey, and Department of Orthopedics, New Jersey Medical School, Rutgers University, Newark, New Jersey; J. Christopher Fritton, Department of Orthopedics, New Jersey Medical School, Rutgers University, Newark, New Jersey; Didier Rajon, Department of Neurosurgery, University of Florida, Gainesville, Florida; and Tom A. Bäck, Department of Radiation Physics, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Source: Read Full Article
