Researchers in the United States have conducted a study showing that Moderna’s coronavirus disease 2019 (COVID-19) vaccine provided durable protection against the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in rhesus macaques.
The delta variant, originally identified in India in October 2020, is currently the dominant strain globally and has been associated with increased transmissibility and more severe COVID-19.
The efficacy of Moderna’s mRNA-1273 against the SARS-CoV-2 delta variant has been shown to wane over time. However, data on the impact that the durability of immune responses has on protection are limited.
Now, a team – from the National Institute of Allergy and Infectious Diseases in Bethesda, Emory University School of Medicine in Atlanta, Bioqual Inc. in Rockville, and Moderna Inc. in Cambridge – has reported on rhesus macaques immunized with two doses of mRNA-1273 and then challenged with the delta variant 48 weeks later.
Robert Seder and colleagues observed that binding and neutralizing antibody titers in the animals’ blood and lower airways decreased during the 48 week post-vaccination period.
At 4 days post-challenge with the delta variant, mRNA-1273-mediated protection in the lungs was durable, but was delayed and potentially dependent on anamnestic antibody responses, says the team.
The findings suggest that rapid and sustained protection against delta in the upper and lower airways may eventually require a booster vaccine.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
Concerns surrounding waning vaccine-induced immunity
The mRNA-based vaccines developed by Pfizer BioNTech and Moderna have been shown to exhibit substantial protection against the vaccine-matched early strain of SARS-CoV-2 –USA-WA1/2020 (WA1).
However, since these vaccines were developed, SARS-CoV-2 variants have emerged with mutations that confer resistance to vaccine-elicited neutralization, raising concerns about the durability of protection provided by mRNA-1273 and other vaccines.
The delta variant, which was first identified in India in October 2020, is now the dominant circulating strain of SARS-CoV-2 globally and has been designated a variant of concern by the World Health Organization.
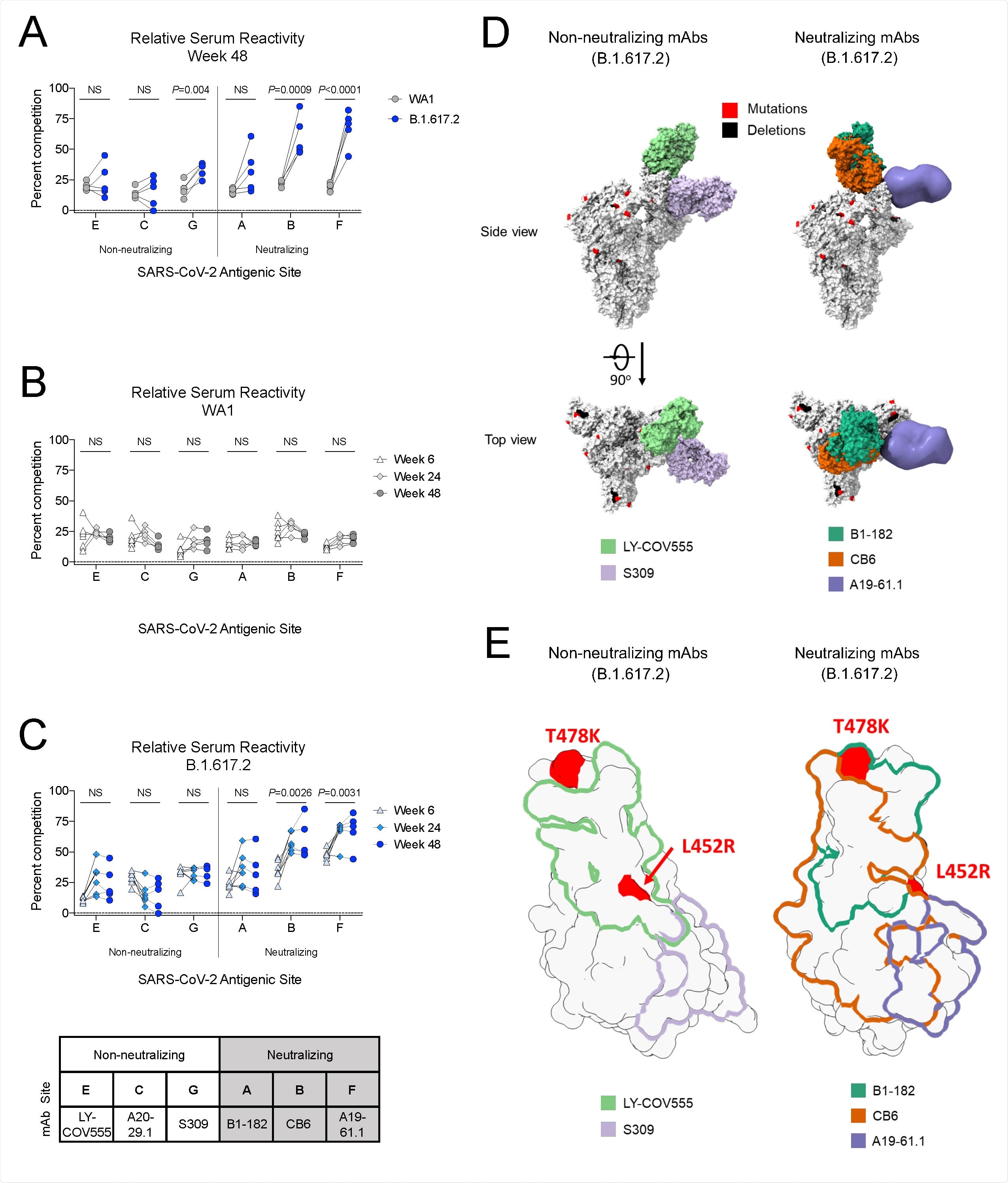
Delta contains mutations in the receptor-binding domain (RBD) of the viral spike protein that contributes to both increased binding to host cell receptors and reduced neutralization by vaccine-elicited antibodies.
Neutralizing antibody titers in mRNA-1273-vaccinated sera have been shown to be reduced 3-fold against the delta variant compared to WA1 shortly after immunization.
However, recent studies in the UK, United States, and Qatar have also shown that although mRNA-based vaccines appear to be three times less effective at protecting against asymptomatic and symptomatic B.1.617.2 infection, the protective efficacy against severe disease is not reduced.
“Furthermore, there is no analysis of mRNA-1273-elicited immunity out to one year in the context of protection against mild and severe disease in the upper and lower airways,” writes Seder and colleagues.
What did the researchers do?
The team immunized eight rhesus macaques with 100µg mRNA-1273 at weeks 0 and 4 and then infected them with the delta variant 48 weeks later.
To assess potential protection mechanisms, the researchers measured antibody titers in the blood and upper and lower airways following both vaccination and challenge with delta. They compared the findings with eight unvaccinated (control) animals that were also challenged with delta at 48 weeks.
What did the study find?
Serum neutralizing titers against delta significantly decreased over the 48 weeks post-vaccination, with a reciprocal 50% inhibitory dilution (ID50) of 280 at week 6 and 34 at week 48.
Antibody binding titers also decreased in bronchoalveolar lavage (BAL) over this 48 week period.
Four days post-challenge, protection in the lower airway was complete. However, the virus was not culturable in BAL, and viral subgenomic RNA (sgRNA) had declined by around 3-log10, compared with the control animals.
Importantly, a striking anamnestic antibody response was observed in the lower airways. The geometric mean titer against delta increased 590-fold by day 4 post-challenge, compared with the pre-challenge timepoint, and by day 7 no sgRNA was detected in six of the eight vaccinated animals.
The researchers say these findings suggest that protection in the lower airway is durable but somewhat delayed and may depend on a recall antibody response.
In nasal swabs, sgRNA had declined 1-log10 and the virus remained culturable, suggesting that a higher threshold of antibody is required for protection in the upper airway, compared with the lower airway.
The team says these data are consistent with studies in human vaccinees showing that immunization can provide a significantly higher level of protection against severe disease than mild or asymptomatic infection.
What are the implications?
Seder and colleagues say the study findings suggest that the mRNA-1273 vaccine provides durable protection against delta in the lower airways, likely through anamnestic induction of antibody responses in the lungs.
However, they add that it is important to consider that control of the virus was both limited in the nose and briefly delayed in the lungs. This could provide the virus with a more significant opportunity for transmission, particularly if variants emerge that are more transmissible, more virulent or more resistant to neutralization.
The researchers advise that in the context of limited virus control in the upper airway and slower kinetics of control in the lower airway, a booster vaccine to increase antibody titers may eventually be warranted.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Seder R, et al. Protection from SARS-CoV-2 Delta one year after mRNA-1273 vaccination in nonhuman primates is coincident with an anamnestic antibody response in the lower airway. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.10.23.465542, https://www.biorxiv.org/content/10.1101/2021.10.23.465542v1
Posted in: Medical Research News | Disease/Infection News
Tags: Allergy, Antibodies, Antibody, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Efficacy, immunity, Immunization, Infectious Diseases, Lungs, Medicine, Monoclonal Antibody, Protein, Receptor, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
