A novel antibody-drug conjugate continues to demonstrate superior benefit for patients with HR+, HER2- metastatic breast cancer when compared to standard chemotherapy, according to a new study in The Lancet.
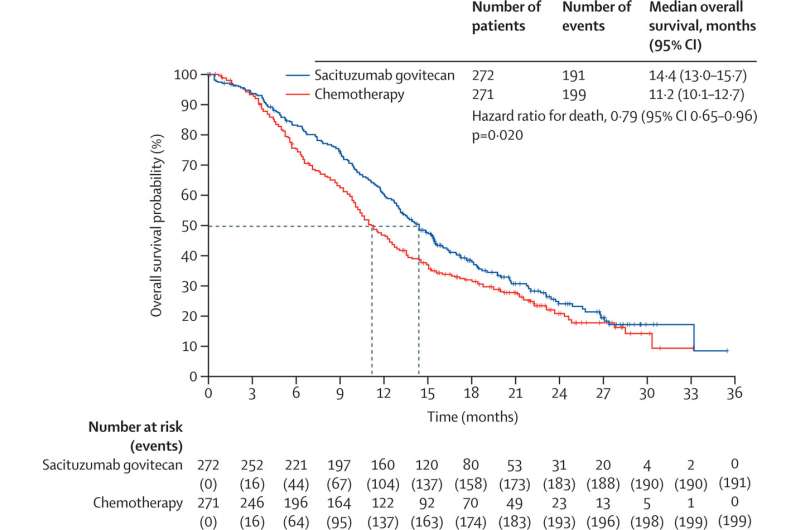
In the second planned analysis of data from the Phase 3 TROPiCS-02 clinical trial, published today, investigators report that sacituzumab govitecan produced a statistically significant improvement in overall survival compared to chemotherapy. The overall survival for patients taking part in the global study was 14.4 months for those who received sacituzumab govitecan and 11.2 months for those given chemotherapy.
The sacituzumab group also showed superior response rates as well as prolonged median progression-free survival when compared to standard chemotherapy. Patients treated with sacituzumab had a 34% lower risk of cancer progression or death than those treated with chemotherapy.
“These data reinforce that sacituzumab govitecan is leading to improvements in both progression- free and overall survival and that patients saw benefit irrespective of their tumor’s Trop-2 expression,” says Sara Tolaney, MD, MPH, chief of the Division of Breast Oncology at the Susan F. Smith Center for Women’s Cancers at Dana-Farber and the study’s senior author. “This updated analysis continues to support sacituzumab govitecan as a standard treatment for patients with pretreated, endocrine-resistant HR+/HER2- metastatic breast cancer.”
Earlier data from the TROPICs-02 study that was presented at last year’s ASCO annual meeting as well at the European Society for Medical Oncology conference led to the accelerated FDA approval of sacituzumab govitecan for the treatment of metastatic HR+/HER2- breast cancer in February of this year.
The first authors of the study are Hope S. Rugo, MD, of the University of California-San Francisco and the Helen Diller Family Comprehensive Cancer Center, and Aditya Bardia, MD, MPH, of Massachusetts General Hospital Cancer Center.
Sacituzumab govitecan is made up of an antibody that targets Trop-2, a cell receptor abundant in many types of solid tumors, including breast cancer, and a cancer drug called SN-38. It has been approved by the FDA for certain patients with triple-negative breast cancer and showed encouraging activity in an early-phase clinical trial in patients whose HR+/HER2- metastatic breast cancer had progressed after at least two prior chemotherapies.
The TROPICS-02 clinical trial involved 543 patients with metastatic HR+/HER2- breast cancer who previously had been treated with endocrine therapy and chemotherapy. Half were randomly assigned to receive sacituzumab govitecan and half received chemotherapy.
The most common adverse effects of treatment were neutropenia (a low count of neutrophils, a type of white blood cell), diarrhea, nausea, alopecia (hair loss), fatigue, and anemia, all of which occurred at a higher rate in patients in the sacituzumab govitecan group than the chemotherapy group.
More information:
Hope S Rugo et al, Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial, The Lancet (2023). DOI: 10.1016/S0140-6736(23)01245-X
Journal information:
The Lancet
Source: Read Full Article