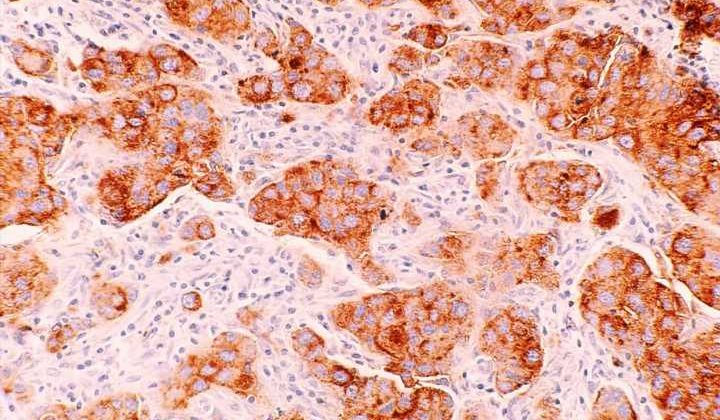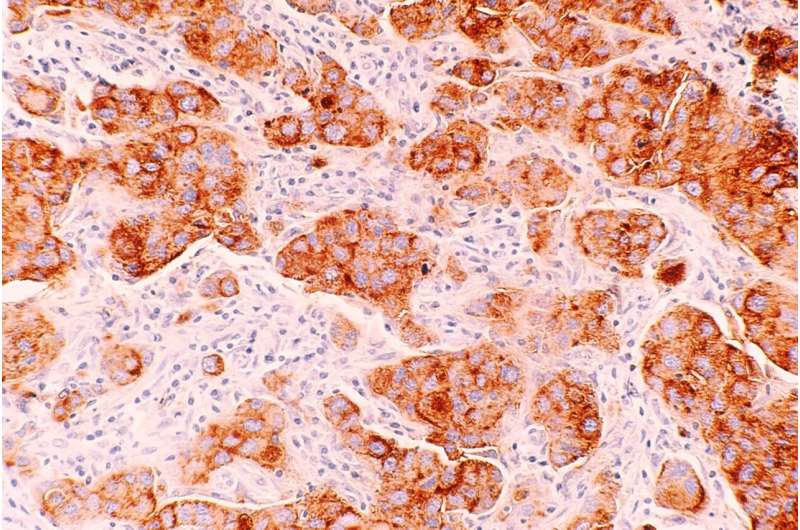
A new study published in Science Translational Medicine has found that in women with BRCA gene mutations, having a high body mass index (BMI) is linked to more DNA damage in breast cells. Specifically, the study discovered that elevated BMI was associated with more damage to epithelium tissue DNA. They also found connections between the damage and increased estrogen production as well as hormones related to obesity, such as leptin and insulin.
Lead author Priya Bhardwaj, Ph.D. Candidate at Weill Cornell Medicine, and her team worked with cultured healthy breast tissues collected from 69 individuals with mutated BRCA1 and BRCA2 genes. The samples were categorized as coming from a donor of lower weight (BMI < 25 kg/m2, n = 43) or overweight/obese (BMI ≥ 25.0 kg/m2, n = 26).
In the lab, they found they could reduce elevated BMI’s effects on epithelial cell DNA damage by blocking estrogen biosynthesis or using the drug fulvestrant to blunt estrogen receptor activity. Researchers also found that inhibiting leptin and insulin signaling in the tissues reduced DNA damage, illustrating potential causal links between obesity, hormone activity, and epithelium DNA health. Since damaged DNA is already considered a causal factor for cancer to develop, the research highlights an essential accelerator of risk and hints at possible drug mitigation.
BReast CAncer genes
BRCA1 and BRCA2 genes are a dynamic duo of which everyone has some version. BRCA genes work together, protecting people from getting certain cancers by promoting mutation-free cell division and repairing DNA damage in cells. Effectively preventing errors from being reproduced by damaged cells prevents cancerous tumors from forming. Mutations in these genes are so commonly associated with breast cancer that they have the unfortunate fate of being named after the very disease they usually work to suppress.
There are tens of thousands of BRCA variants, most of which do their job well, repairing DNA and keeping us tumor free. However, certain variants of these genes can increase the risk that they get the repairs wrong. A bad DNA repair job can quickly become a mutated cell born without its cell signaling genes intact, leading to mutated tissues with an unregulated appetite that ignores requests by surrounding tissues to stop growing, becoming a tumor—and a cancer diagnosis.
The study led by Bhardwaj shows that in addition to potential bad repair jobs by cancer-related BRCA variants, people with elevated BMI run a more significant risk of needing repairs because of increased damage mechanisms. The researchers suggest that maintaining a lower body weight or pharmacologically targeting estrogen or metabolic dysfunction may reduce the risk of breast cancer in this already high-risk population.
An additional experiment on mice was included in the study. In that experiment, mice were fed a high-fat or low-fat diet over 28 weeks. In that side experiment, the high-fat fed mice had elevated mammary gland DNA damage compared with low-fat fed mice. The high-fat fed group was found to be 16.5% more likely to develop mammary tumors by the end of the surveillance period.
More information:
Priya Bhardwaj et al, Obesity promotes breast epithelium DNA damage in women carrying a germline mutation in BRCA1 or BRCA2, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.ade1857
Journal information:
Science Translational Medicine
Source: Read Full Article