Cardiovascular diseases account for 32% of global deaths. Myocardial infarction, or heart attacks, play a large part in heart diseases and the necrosis of cardiac tissue after blood supply is decreased or stopped.
In APL Bioengineering, researchers from Pohang University of Science and Technology in South Korea take stock of stem cell-laden 3D-bioprinted cardiac patch technologies and their efficacy as a therapeutic and regenerative approach for ischemic cardiomyopathy in reversing scar formation and promoting myocardial regeneration.
“Currently available therapeutics are not sufficient for the complete treatment of myocardial infarction,” said author Jinah Jang. “The development of a new, advanced modality, such as reducing adverse cardiac remodeling, promoting myocardial functions, and correcting molecular or genetic defects, is urgently required.”
The researchers explore various types of candidate stem cells that possess cardiac regenerative potential, explaining their applications and limitations. They share updates on the challenging implementation of the state-of-the-art 3D-bioprinting approach to fabricate a cardiac patch and highlight different strategies to implement vascularization and augment cardiac functional properties with respect to electrophysiological similarities to native tissue.
Following a myocardial infarction, myocardial tissues and vasculatures are equally and severely damaged. Therefore, therapeutic or regenerative approaches should be planned to target both of them concurrently to achieve a successful cardiac repair, because the heart has very little ability to regenerate cardiomyocytes or heart cells by itself.
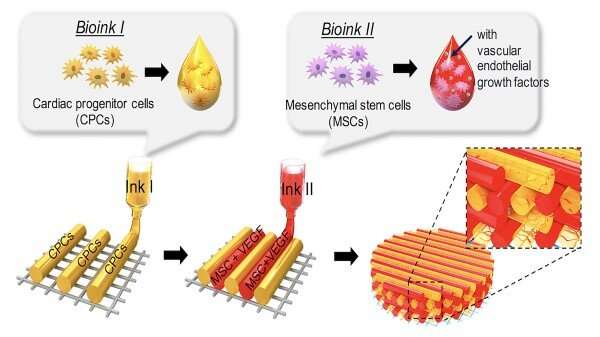
Employing a 3D-bioprinting strategy to geometrically control the spatial patterning and using dual stem cell therapy as its co-culture can play an important role in promoting and synergistically improving vascularization as well as cardiac function following myocardial infarction.
Currently applied patch-based stem cell therapies have shown advanced efficacy, rather than using single-component therapies, by providing a tissue-friendly environment during the time of host-graft integration.
“It would be helpful for tracing cells of the printed patch to investigate the mode of action for the transplanted patch,” said author Sanskrita Das.
“Although there are still inherent limitations for the clinical study, the suggested stem cell delivery platform technology provides a practical therapeutic perspective for various tissue engineering applications,” said author Hyoryung Nam.
Source: Read Full Article
