
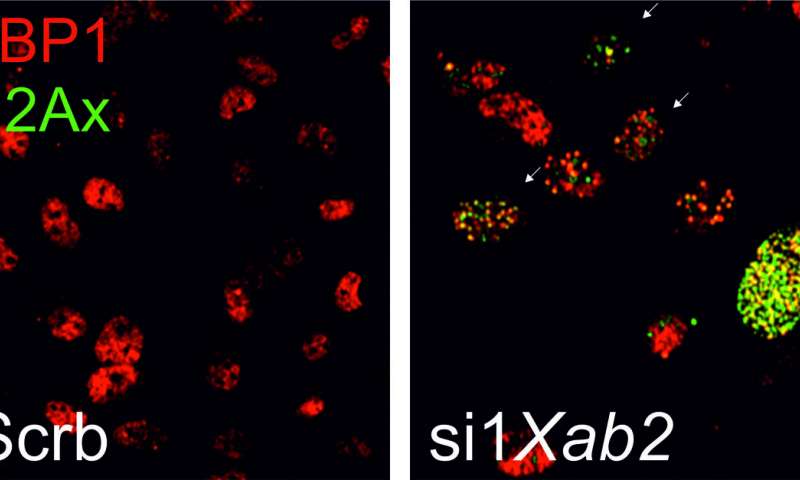
Over time, we accumulate genetic damage that accelerates the aging process, increasing the risk of carcinogenesis. DNA is continually challenged by genotoxic factors that affect its fragile structure, inhibiting cell functions. To meet this challenge, cells have evolved a number of overlapping DNA repair mechanisms that detect and repair DNA damage. Nucleotide excision repair (NER) is a major DNA repair mechanism that cells employ to remove a wide class of bulky, DNA-distorting lesions from the genome. The importance of NER defects in man is illustrated by rare syndromes that either show increased cancer predisposition or dramatic features of accelerated aging, including depletion of fat depots. However, with the exception of cancer and aging, the links between defects in NER and the rapid onset of developmental defects in humans are not well understood.
Researchers at the Foundation for Research & Technology-Hellas (FORTH) have revealed that DNA repair and RNA processing are functionally interconnected and that inborn defects in RNA processing or DNA repair associate with progeria, the metabolic syndrome, neurodegeneration and cancer. Their findings provide evidence that the XAB2 protein is involved in repairing genetic damage, as well as, in the vital process of regulating gene expression that affects the production of other proteins, depending on the needs of each cell. Mutations in the gene that activates the production of XAB2 could even lead to stillbirth. The research also explains why mutations in genes involved in repairing genetic damage lead to developmental abnormalities in humans.
The current research, which was recently published in the prestigious journal Nature Communications, was conducted by Dr. Evi Goulielmaki and Dr. Maria Tsekrekou, with the group leader Prof. George Garinis (Dep. of Biology of the University of Crete), affiliated researcher at the Institute of Molecular Biology and Biotechnology of FORTH, who explained:
Source: Read Full Article
