As newer vaccines are being developed to combat the ongoing pandemic of coronavirus disease 2019 (COVID-19), targeting the pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a new preprint research paper posted to the bioRxiv* server describes a new subunit nanovaccine that appears to have robust immunogenicity and neutralizing activity.
New approaches to spike-based vaccines
Both the viral spike antigen and its receptor-binding domain (RBD), which elicit highly potent neutralizing antibodies to the virus have been used to create most vaccines in use and under development at present. The RBD is more straightforward to produce and more stable than the full-length spike protein, but the latter is more immunogenic.
However, newer approaches to RBD assembly have significantly improved its ability to induce a neutralizing response. One such is the induction of RBD self-assembly to form virus-like particles (VLPs).
The current study aimed to improve cellular and adaptive immunity induced by the viral RBD since both CD4+ and CD8+ T cells are key to virus clearance and memory immune cell responses for long-term immunity.
Secondly, the study seeks to compare the efficacy of such nanoparticle assemblies with more conventional vaccines.
Polymersomes with RBD antigen display
The researchers drew on their earlier experience of polymersomes (PS), which are self-assembled bodies formed from the block copolymer poly(ethylene glycol)-bl-poly(propylene sulfide) (PEG-PPS). These were proved to effectively deliver antigen and adjuvant to the endosomes of dendritic cells. These are innate immune cells that present the antigens to responsive immune cells of the adaptive immune system.
Within the endolysosomes, the PPS undergoes oxidation, and the PS thereby forms micelles instead, releasing the encapsulated antigen or other cargo. The result has been dendritic cell activation, a strong T cell response, and high polyclonal antibody titers.
The researchers attempted to enhance the antibody response to PS-borne antigens without sacrificing the cellular immune response by modifying them in such a way that they would resemble a viral particle in form, displaying multiples of the target antigen.
The hope was that this multivalent RBD display would improve crosslinking characteristics and B cell receptor (BCR) clustering to induce higher levels of neutralizing antibodies.
In this study, PS with both surface-bound RBD and encapsulated RBD were evaluated with an adjuvant monophosphoryl lipid A-encapsulated PS (MPLA PS).
The researchers produced their nanovaccine candidate by conjugating spike antigens to the PS surface, using a versatile N3-PEG-PPS platform that can be adapted for any antigen. It is capable of being assembled to form PS particles with multiple functional groups on the surface that can attach the desired antigen – in this case, the spike RBD.
PS, which encapsulates the RBD were also formulated, along with that encapsulating the MLPA adjuvant. They confirmed the persistence of biological activity in the conjugated RBD, with a high binding affinity for the host cell receptor, angiotensin-converting enzyme 2 (ACE2). Conversely, unbound PS failed to bind to the receptor.
The adjuvant used, MPLA, was confirmed to enhance pro-inflammatory pathways and activate antigen-presenting cells (APCs).
Surface-RBD PS
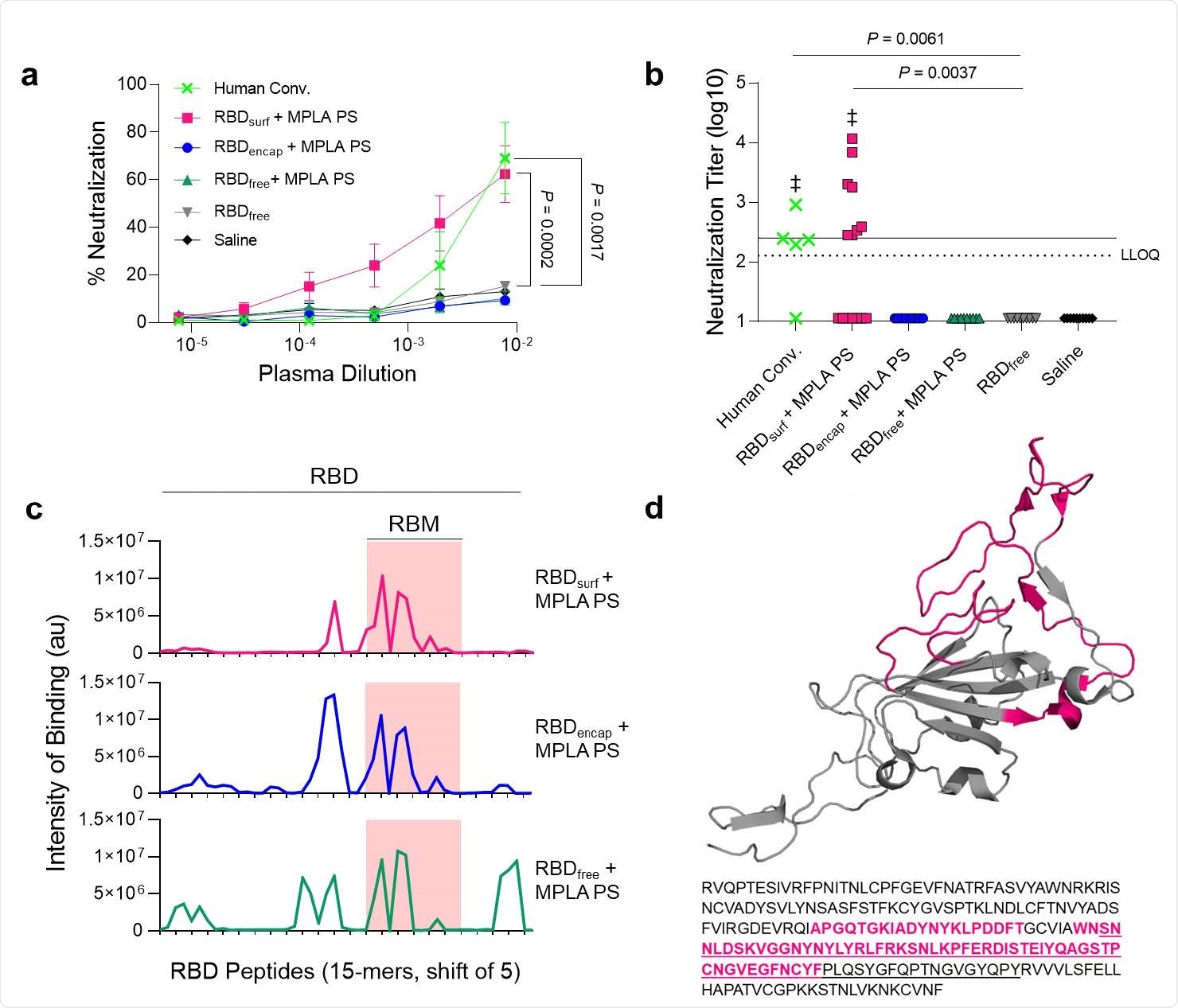
When tested in preclinical studies, the results show that mice vaccinated with two doses of the surface-RBD display formulation developed high titers of neutralizing antibodies to SARS-CoV-2, along with strong germinal center responses indicating B cell activation and robust CD4+ and CD8+ T cell immunity.
The antibody response began less than one week from the first dose, and the highest response was with the encapsulated RBD formulation. The levels remained constant or slowly increased until the boost dose, at which point it went up by 1.3-1.6-fold.
The antibody response to the surface RBD was dominated by IgG1, compared to IgG2 and IgG3, also indicating a Th2-biased response, whereas it was more balanced with the encapsulated form.
IgA antibodies were detectable in all groups that received adjuvanted vaccines, which is key to producing mucosal immunity.
Neutralizing antibodies were induced by the RBD-conjugated antigen more effectively, as shown by a cell-based assay, with a high viral neutralization titer (VNT) – the at which 50% of cells survive following virus challenge.
The neutralizing capacity appears to be equivalent to that of high-titer convalescent plasma.
Encapsulated RBD PS
In contrast, the encapsulated RBD antigen showed effective recognition of a broader range of linear epitopes. However, when used together, the combination led to a high titer of RBD-directed immunoglobulin (Ig) G antibodies but without significantly increased neutralizing activity beyond baseline levels.
The reason appears to be that the surface-conjugated RBD antibodies bind with high affinity to epitopes within the receptor-binding motif (RBM) of the RBD, but the encapsulated RBD produces antibodies with greater epitope diversity outside the RBM.
Effect of MPLA adjuvant
Once the adjuvant was added, all groups showed higher T follicular helper cells in the lymph nodes draining the injection site at one week from vaccination, with activation markers.
The mice that received the adjuvanted vaccines also showed evidence of stronger germinal center activation. This is associated with class switching and the differentiation of long-lived plasma cells and class-switched memory B cells with high affinity for the target antigen, or short-lived plasmablasts and IgM memory cells.
Within the B cell population with RBD specificity, a better response was observed with the surface-conjugated RBD vaccine.
These mice not only show strong specific antibody responses but also higher Th1-skewed CD4+ and CD8+ T cell responses compared to those that received free RBD, with or without the adjuvant.
Superiority of surface-display RBD PS
“The differences in the immune responses elicited by the two PS antigen formulations suggest that surface display of antigen leads to stronger GC responses, while PS-encapsulated antigen elicits more predominantly an extrafollicular response.”
A GC response is preferable to extrafollicular responses in that it results in B cells with higher affinity and a more durable immune response. The factors responsible for this more functional antibody response include the multimeric display of the RBD antigen along with its higher exposure to B cells.
The Th1-type trend of immune response observed here is probably due to the adjuvant used in this vaccine candidate and predicts a lower risk of adverse events related to the vaccine.
What are the implications?
The polymersome presentation produces improved antigen presentation to CD8+ T cells. Firstly, these nanocarrier molecules target the APCs, which are designed to take up molecules to which the membrane is impermeable, via micropinocytosis.
Secondly, the endosomes release antigen more efficiently, especially with the encapsulated RBD PS. These release the antigen readily with a low level of oxidation, without acidification.
Both adjuvant and antigen can be delivered on the same PS particle.
Both types of RBD-bearing PS were stable for 6 months or more at 4 °C, thus making them ideal for large-scale vaccine distribution in low-resource settings. Moreover, a single PS can be used to conjugate multiple antigens from the same or different viruses to elicit cross-reactive neutralizing antibodies.
The use of antigens in PS formulations could benefit the design of future vaccines where T cell responses are essential, including cancer vaccines and those against viruses like hepatitis C and HIV.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Volpatti, L. R. et al. (2021). Polymersomes decorated with SARS-CoV-2 spike protein receptor binding domain elicit robust humoral and cellular immunity. bioRxiv preprint. doi: https://doi.org/10.1101/2021.04.08.438884. https://www.biorxiv.org/content/10.1101/2021.04.08.438884v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Amino Acid, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Antigen, Assay, binding affinity, Cancer, CD4, Cell, Cell Death, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, Dendritic Cell, Efficacy, Enzyme, Hepatitis C, HIV, Immune Response, Immune System, Immunoglobulin, in vitro, Lymph Nodes, Nanoparticle, Pandemic, Pathogen, Peptides, Polyclonal Antibody, Preclinical, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article
